AWMSG Medicines Assessment Process for Licensed and Off-label Medicines
Improving access to medicines where there is a clinical need or benefit to the NHS in Wales and the people it serves.
Background
Since 2002, the All Wales Medicines Strategy Group (AWMSG) has provided health technology assessment (HTA) :
- on medicines that have not been included in the National Institute for Health and Care Excellence (NICE) work programme; or
- if there might be a benefit in AWMSG assessing a medicine before NICE.
After assessment, AWMSG’s recommendations are ratified by Welsh Government and published as 'AWMSG advice'.
In 2015, the One Wales Medicines process (previously known as the One Wales Interim Commissioning process) was introduced to give an alternative way to assess and allow access to medicines for clearly defined and specific cohorts (groups) of patients in the absence of HTA by NICE or AWMSG.
HTA is the gold standard for evaluating the clinical effectiveness and cost-effectiveness of a medicine. However, sometimes a medicine with a service benefit or patient need might fall outside the established scope for HTA, by NICE or by AWMSG, and the medicine does not meet the current criteria for assessment through the One Wales Medicines process. This may include medicines that are routinely available elsewhere in the UK through routes of access that do not apply in Wales.
In 2024, AWMSG adapted and updated their existing medicine assessment processes into one overarching process to support an ‘All-Wales’ approach to medicine access to ensure that the needs of the NHS in Wales are met.
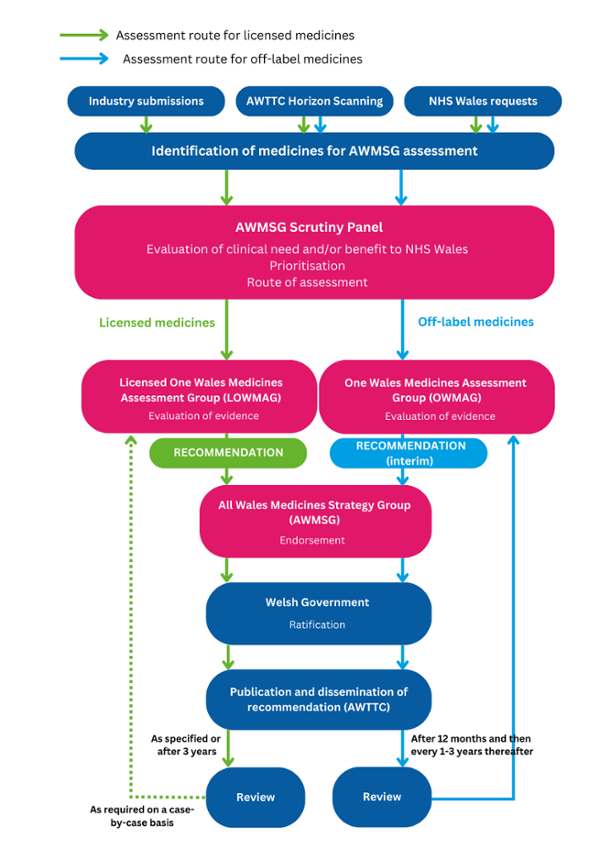
Summary of the updated AWMSG medicine assessment process
The All Wales Therapeutics and Toxicology Centre (AWTTC) supports AWMSG in providing evidence-based assessment processes. Medicines are identified for assessment through the AWTTC horizon scanning process, which includes engaging with key stakeholders:
- healthcare professionals;
- the pharmaceutical industry;
- patient organisations;
- patients, their families and carers; and
- the general public.
Once a licensed medicine or an off-label medicine is identified, the AWMSG Scrutiny Panel will use pre-defined criteria to decide if the medicine is suitable for assessment by AWMSG. AWMSG’s two sub-groups: the Licensed One Wales Medicines Assessment Group (LOWMAG), and the One Wales Medicines Assessment Group (OWMAG), will assess and make a recommendation for AWMSG to endorse and Welsh Government to ratify.
LOWMAG replaces the New Medicines Group (NMG), and considers and provides recommendations on medicines that are licensed. OWMAG considers and provides recommendations on off-label medicines. Once ratified by Welsh Government, these recommendations will ensure equitable and consistent funding of these medicines across the NHS in Wales.
An Equality and Health Impact Assessment (EHIA) for the AWMSG Medicines Assessment Process for Licensed and Off-label Medicines has been completed and key actions have been identified.
A visual representation of the updated process is shown below and can be downloaded as a PDF document.
Identification of medicines for AWMSG assessment flowchart